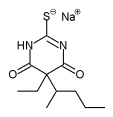
Sodium thiopental
 |
|
|---|---|
 |
|
| Systematic (IUPAC) name | |
| (RS)-[5-ethyl-4,6-dioxo-5-(pentan-2-yl)-1,4,5,6-tetrahydropyrimidin-2-yl]sulfanide sodium | |
| Identifiers | |
| CAS number | 71-73-8 (sodium salt) 76-75-5 (free acid) |
| ATC code | N01AF03 N05CA19 |
| PubChem | CID 3000714 |
| DrugBank | DB00599 |
| ChemSpider | 2272257 |
| Chemical data | |
| Formula | C11H17N2NaO2S |
| Mol. mass | 264.32 g/mol |
| Pharmacokinetic data | |
| Half-life | 5.5[1]-26 hours[2] |
| Therapeutic considerations | |
| Pregnancy cat. | ? |
| Legal status | Schedule III (US) |
| Routes | Oral, intravenous |
| |
|
Sodium thiopental, better known as Sodium Pentothal (a trademark of Abbott Laboratories), thiopental, thiopentone sodium, or Trapanal (also a trademark), is a rapid-onset short-acting barbiturate general anaesthetic. Sodium thiopental is a depressant and is sometimes used during interrogations – not to cause pain (in fact, it may have just the opposite effect), but to weaken the resolve of the subject and make him more compliant to pressure.[3] Thiopental is a core medicine in the World Health Organization's "Essential Drugs List", which is a list of minimum medical needs for a basic healthcare system.[4]
Contents |
Barbiturates
Barbiturates are a class of drugs that act on the GABAA receptor in the brain and spinal cord. The GABAA receptor is an inhibitory channel that decreases neuronal activity, and barbiturates enhance the inhibitory action of the GABAA receptor. Barbiturates, benzodiazepines, and alcohol all bind to the GABAA receptor. Barbiturates that act on the barbiturate binding site of the GABAA receptor directly gate the chloride ion channel of the GABAA receptor, whereas benzodiazepines acting on the benzodiazepine site on the GABAA receptor increase the opening frequency of the chloride ion channel. This explains why overdoses of barbiturates may be lethal whereas overdoses of benzodiazepines alone are typically not lethal. Another explanation is that barbiturates can activate GABA receptors in the absence of the GABA molecule, whereas benzodiazepines need GABA to be present to have an effect: this may explain the more widespread effects of barbiturates in the central nervous system. Barbiturates have anesthetic, sedative, anxiolytic, anticonvulsant and hypnotic properties. Barbiturates do not have analgesic effects.[5]
Further, barbiturates are relatively "promiscious" (i.e. non-selective) compounds that bind to an entire superfamily of ligand-gated ion channels, of which the GABAA receptor channel is only one of several representatives. This superfamily of ion channels includes the neuronal nACHR channel, the 5HT3R channel, the GlyR channel and others. Surprisingly, while GABAA receptor currents are increased by barbiturates (and other general anaesthetics), ligand-gated ion channels that are predominantly permeable for cationic ions are blocked by these compounds. For example, neuronal nACHR channels are blocked by clinically relevant anaesthetic concentrations of both thiopental and pentobarbital.[6] Such findings implicate (non-GABA-ergic) ligand-gated ion channels, e.g. the neuronal nAChR channel, in mediating some of the (side) effects of barbiturates.[7]
Uses
Anesthesia
Thiopental is an ultra-short-acting barbiturate and has been used commonly in the induction phase of general anesthesia. Its use in the United States and elsewhere has been largely replaced with that of propofol. Following intravenous injection the drug rapidly reaches the brain and causes unconsciousness within 30–45 seconds. At one minute, the drug attains a peak concentration of about 60% of the total dose in the brain. Thereafter, the drug distributes to the rest of the body and in about 5–10 minutes the concentration is low enough in the brain such that consciousness returns.
A normal dose of thiopental (usually 4–6 mg/kg) given to a pregnant woman for operative delivery (caesarian section) rapidly makes her unconscious, but the baby in her uterus remains conscious. However, larger or repeated doses can depress the baby.
Thiopental is not used to maintain anesthesia in surgical procedures because, in infusion, it displays zero-order elimination kinetics, leading to a long period before consciousness is regained. Instead, anesthesia is usually maintained with an inhaled anesthetic (gas) agent. Inhaled anesthetics are eliminated relatively quickly, so that stopping the inhaled anesthetic will allow rapid return of consciousness. Thiopental would have to be given in large amounts to maintain an anesthetic plane, and because of its 11.5–26 hour half-life, consciousness would take a long time to return.[8]
In veterinary medicine, thiopental is also used to induce anesthesia in animals. Since thiopental is redistributed to fat, certain breeds of dogs – primarily the sight hounds – can have prolonged recoveries from thiopental due to their lack of body fat and their lean body mass. Thiopental is always administered intravenously, as it can be fairly irritating; severe tissue necrosis and sloughing can occur if it is injected incorrectly into the tissue around a vein.
Medically induced coma
In addition to anesthesia induction, thiopental was historically used to induce medical comas. It has now been superseded by drugs such as propofol.
Thiopental has a long Context Sensitive Half Time (CSHT), meaning infusions saturate peripheral compartments (fat, muscle etc.). When the infusion is stopped, the drug redistributes from the peripheral tissues back into the blood, prolonging the effect.
Thiopental also exhibits zero order kinetics at higher doses. The rate of elimination becomes constant.
Patients with brain swelling, causing elevation of the intracranial pressure, either secondary to trauma or following surgery, may benefit from this drug. Thiopental, and the barbiturate class of drugs, decrease neuronal activity and therefore decrease the production of osmotically active metabolites, which in turn decreases swelling. Patients with significant swelling have improved outcomes following the induction of coma. Reportedly, thiopental has been shown to be superior to pentobarbital[9] in reducing intracranial pressure.
Euthanasia
Thiopental is used intravenously for the purposes of euthanasia. The Belgians and the Dutch have created a protocol that recommends sodium thiopental as the ideal agent to induce coma, followed by pancuronium bromide.[10]
Intravenous administration is the most reliable and rapid way to accomplish euthanasia and therefore can be safely recommended. A coma is first induced by intravenous administration of 20 mg/kg thiopental sodium (Nesdonal) in a small volume (10 ml physiological saline). Then a triple intravenous dose of a non-depolarizing neuromuscular muscle relaxant is given, such as 20 mg pancuronium dibromide (Pavulon) or 20 mg vecuronium bromide (Norcuron). The muscle relaxant should preferably be given intravenously, in order to ensure optimal availability. Only for pancuronium dibromide (Pavulon) are there substantial indications that the agent may also be given intramuscularly in a dosage of 40 mg.[10]
Lethal injection
Along with pancuronium bromide and potassium chloride, thiopental is used in 34 states of the U.S. to execute prisoners by lethal injection. A very large dose is given, placing the subject into a rapidly induced coma. Executions using the three drug combination are usually effective in approximately 10 minutes, but have been known to take several times this amount of time. The use of thiopental alone is hypothesized to cause death in approximately 45 minutes.[11] The use of sodium thiopental has been the cause of current Supreme Court challenges to the lethal injection protocol, after a study in the medical journal The Lancet, where autopsy studies on executed inmates revealed that there was not a high enough concentration of thiopental in their blood to have caused unconsciousness.
On December 8, 2009, the State of Ohio became the first to use a single dose of sodium thiopental for its capital execution, following the failed use of the standard three-drug cocktail during a recent execution, due to inability to locate suitable veins. Kenneth Biros was executed using the single-drug method. The execution took 43 minutes and was concluded at 11:47 a.m., including approximately 30 minutes to complete the process of inserting the needle, and about 10 minutes between administering the single dose and the pronouncement of death.[12][13]
The state of Ohio executed a second man using sodium thiopental on January 7, 2010. Vernon Smith was pronounced dead 8 minutes after the time of injection.[14]
The state of Ohio executed a third man using sodium thiopental on April 20, 2010. Daryl Durr was pronounced dead at 10:36 am for the 1988 murder of a 16-year-old girl, Angel Vincent.[15]
Most recently, William Garner was executed in Ohio with sodium thiopental for the 1992 murders of 5 children. [16]
Truth serum
Thiopental (Pentathol) is still used in some places as a truth serum. The barbiturates as a class decrease higher cortical brain functioning. Some psychiatrists hypothesize that because lying is more complex than telling the truth, suppression of the higher cortical functions may lead to the uncovering of the "truth". However, the reliability of confessions made under thiopental is dubious; the drug tends to make subjects chatty and cooperative with interrogators, but a practiced liar or someone who has a false story firmly established could still relate the falsehood while under the influence of the drug.[17]
Psychiatry
Psychiatrists have used thiopental to desensitize patients with phobias,[18] and to "facilitate the recall of painful repressed memories."[19] One psychiatrist who worked with thiopental is the Dutch Professor Jan Bastiaans, who used this procedure to help release trauma in victims of the Nazis.[20]
Metabolism
As with all lipid-soluble anaesthetic drugs, the short duration of action of STP is due almost entirely to its redistribution away from central circulation towards muscle and fat tissue. Once redistributed, the free fraction in the blood is metabolised in the liver. Sodium thiopental is mainly metabolized to pentobarbital,[21] 5-ethyl-5-(1'-methyl-3'-hydroxybutyl)-2-thiobarbituric acid, and 5-ethyl-5-(1'-methyl-3'-carboxypropyl)-2-thiobarbituric acid.[22]
Dosage
The usual dose range for induction of anesthesia using thiopental is from 3 to 7 mg/kg; however, there are many factors that can alter this. Premedication with sedatives such as benzodiazepines or clonidine will reduce requirements, as do specific disease states and other patient factors. Among patient factors are: age, sex, lean body mass. Specific disease conditions that can alter the dose requirements of thiopentone and for that matter any other intravenous anaesthetic are: hypovolemia, burns, azotemia, hepatic failure, hypoproteinemia, etc.
Side effects
As with nearly all anesthetic drugs, thiopental causes cardiovascular and respiratory depression resulting in hypotension, apnea and airway obstruction. For these reasons, only suitably trained medical personnel should give thiopental in an environment suitably equipped to deal with these effects. Side effects include headache, emergence delirium, prolonged somnolence, and nausea. Intravenous administration of sodium thiopental is followed instantly by an odor and/or taste sensation, sometimes described as being similar to rotting onions, or to garlic. The hangover from the side effects may last up to 36 hours.
Although individual molecules of thiopental contain one sulfur atom, it is not a sulfonamide, and does not show allergic reactions of sulfa/sulpha drugs.
Drug interaction
Co-administration of pentoxifylline and thiopental causes death by acute pulmonary oedema in rats. This pulmonary oedema was not mediated by cardiac failure or by pulmonary hypertension but was due to increased pulmonary vascular permeability.[23]
History
Sodium thiopental was discovered in the early 1930s by Ernest H. Volwiler and Donalee L. Tabern, working for Abbott Laboratories. It was first used in human beings on March 8, 1934, by Dr. Ralph M. Waters[24] in an investigation of its properties, which were short-term anesthesia and surprisingly little analgesia.[25] Three months later,[26] Dr. John S. Lundy started a clinical trial of thiopental at the Mayo Clinic at the request of Abbott.[27]
Thiopental is famously associated with a number of anesthetic deaths in victims of the attack on Pearl Harbor. These deaths, relatively soon after the substance's discovery, were due to excessive doses given to shocked trauma patients. Evidence has become available through freedom of information legislation and has been reviewed in the "British Journal of Anaesthesia".[28] Thiopental anaesthesia was in its early days, but nevertheless only 13 of 344 wounded admitted to the Tripler Army Hospital did not survive.
Thiopental is still rarely used as a recreational drug, usually stolen from veterinarians or other legitimate users of the drug; however, more common sedatives such as benzodiazepines are usually preferred as recreational drugs, and abuse of thiopental tends to be uncommon and opportunistic.
References
- ↑ Russo H, Brès J, Duboin MP, Roquefeuil B (1995). "Pharmacokinetics of thiopental after single and multiple intravenous doses in critical care patients". Eur. J. Clin. Pharmacol. 49 (1-2): 127–37. doi:10.1007/BF00192371. PMID 8751034.
- ↑ Morgan DJ, Blackman GL, Paull JD, Wolf LJ (June 1981). "Pharmacokinetics and plasma binding of thiopental. II: Studies at cesarean section". Anesthesiology 54 (6): 474–80. doi:10.1097/00000542-198106000-00006. PMID 7235275.
- ↑ Sydney Morning Herald, Truth serum used on 'serial child killers', January 12, 2007, Reuters.
- ↑ "WHO Model List of Essential Medicines" (PDF). World Health Organization. March 2005. http://whqlibdoc.who.int/hq/2005/a87017_eng.pdf. Retrieved 2006-03-12.
- ↑ "Anesthesia and Analgesia". University of Virginia School of Medicine. http://www.healthsystem.virginia.edu/internet/ccm/Anesth/aneshome.cfm. Retrieved 2007-08-05.
- ↑ Weber, M; Motin, L; Gaul, S; Beker, F; Fink, RH; Adams, DJ (January 2005). "Intravenous anaesthetics inhibit nicotinic acetylcholine receptor-mediated currents and Ca2+ transients in rat intracardiac ganglion neurons.". British Journal of Pharmacology 144 (1): 98–107. doi:10.1038/sj.bjp.0705942. PMID 15644873.
- ↑ Franks, NP; Lieb, WR (23 November 1998). "Which molecular targets are most relevant to general anaesthesia?". Toxicology Letters 100–101: 1–8. doi:10.1016/S0378-4274(98)00158-1. PMID 10049127.
- ↑ Morgan DJ, Blackman GL, Paull JD, Wolf LJ (1981). "Pharmacokinetics and plasma binding of thiopental. II: Studies at cesarean section". Anesthesiology 54 (6): 474–80. doi:10.1097/00000542-198106000-00006. PMID 7235275.
- ↑ Pérez-Bárcena J, Barceló B, Homar J, et al. (February 2005). "[Comparison of the effectiveness of pentobarbital and thiopental in patients with refractory intracranial hypertension. Preliminary report of 20 patients"] (in Spanish; Castilian). Neurocirugia (Astur) 16 (1): 5–12; discussion 12–3. PMID 15756405. http://www.revistaneurocirugia.com/web/artics/v16n1/1.pdf. Retrieved 2008-07-18.
- ↑ 10.0 10.1 Royal Dutch Society for the Advancement of Pharmacy (1994). "Administration and Compounding of Euthanasic Agents". The Hague. http://wweek.com/html/euthanasics.html. Retrieved 2008-07-18.
- ↑ "Ohio executes inmate with 1-drug lethal injection". AP. December 2009. http://news.yahoo.com/s/ap/20091208/ap_on_re_us/us_ohio_execution. Retrieved 2009-12-08.
- ↑ Martinez, Edecio (8 December 2009). "Kenneth Biros Execution: Ohio Man First to Die Under 1-Drug Thiopental Sodium Method". CBS News. http://www.cbsnews.com/blogs/2009/09/24/crimesider/entry5334823.shtml.
- ↑ "Ohio executes inmate with 1-drug lethal injection". Associated Press. Google. 9 December 2009. Archived from the original on 23 December 2009. http://www.webcitation.org/5mEkZTdUI.
- ↑ Smyth, Julie Carr (7 January 2010). "Ohio executes man in second use of 1-drug method". Houston Chronicle. Associated Press. http://www.chron.com/disp/story.mpl/ap/top/all/6803389.html.
- ↑ "Ohio executes murderer of teen". CNN.com. 20 April 2010. http://www.cnn.com/2010/CRIME/04/20/ohio.execution/index.html?hpt=Sbin.
- ↑ "Man convicted of murdering 5 children executed in Ohio". CNN.com. 13 July 2010. http://www.cnn.com/2010/CRIME/07/13/ohio.execution/index.html?iref=NS1.
- ↑ Anne Bannon; Stevens, Serita Deborah (2007). The Howdunit Book of Poisons (Howdunit). Cincinnati: Writers Digest Books. ISBN 1-58297-456-X.
- ↑ Pearlman, T. (1980). "Behavioral desensitization of phobic anxiety using thiopental sodium". The American Journal of Psychiatry (American Psychiatric Association) 137 (137): 1580–1582. PMID 6108082. http://ajp.psychiatryonline.org/cgi/content/abstract/137/12/1580.
- ↑ "Drugged Future?". TIME. February 24, 1958. http://www.time.com/time/magazine/article/0,9171,863001,00.html.
- ↑ Snelders, Stephen (1998). "The LSD Therapy Career of Jan Bastiaans, M.D.". Newsletter of the Multidisciplinary Association for Psychedelic Studies (Multidisciplinary Association for Psychedelic Studies) 8 (1): 18–20. http://www.maps.org/news-letters/v08n1/08118sne.html.
- ↑ WINTERS WD, SPECTOR E, WALLACH DP, SHIDEMAN FE (July 1955). "Metabolism of thiopental-S35 and thiopental-2-C14 by a rat liver mince and identification of pentobarbital as a major metabolite". J. Pharmacol. Exp. Ther. 114 (3): 343–57. PMID 13243246. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=13243246. Retrieved 2008-07-18.
- ↑ Bory C, Chantin C, Boulieu R, et al. (1986). "[Use of thiopental in man. Determination of this drug and its metabolites in plasma and urine by liquid phase chromatography and mass spectrometry]" (in French). C. R. Acad. Sci. III, Sci. Vie 303 (1): 7–12. PMID 3093002.
- ↑ Pereda J, Gómez-Cambronero L, Alberola A, et al. (October 2006). "Co-administration of pentoxifylline and thiopental causes death by acute pulmonary oedema in rats". Br. J. Pharmacol. 149 (4): 450–5. doi:10.1038/sj.bjp.0706871. PMID 16953192.
- ↑ "This Month in Anesthesia History: March". Anesthesia History Association. http://www.anesthesia.wisc.edu/AHA/Calendar/March.html.
- ↑ Steinhaus, John E. The Investigator and His ‘Uncompromising Scientific Honesty’ American Society of Anesthesiologists. NEWSLETTER. September 2001, Volume 65, Number 9.
- ↑ Imagining in Time—From this point in time: Some memories of my part in the history of anesthesia—John S. Lundy, MD August 1997, AANA Archives-Library
- ↑ History of Anesthesia with Emphasis on the Nurse Specialist Archives of the American Association of Nurse Anesthetists. 1953
- ↑ Bennetts FE (September 1995). "Thiopentone anaesthesia at Pearl Harbor". Br J Anaesth 75 (3): 366–8. PMID 7547061. http://bja.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=7547061. Retrieved 2008-07-18.
External links
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||